Unraveling the roles of ADP-ribosylation in physiology and disease
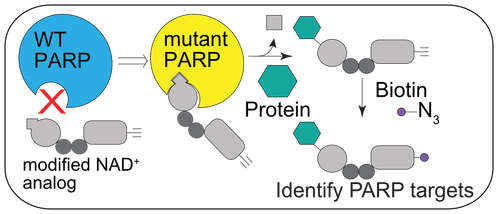
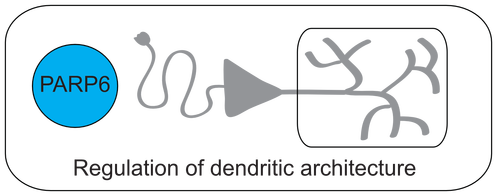
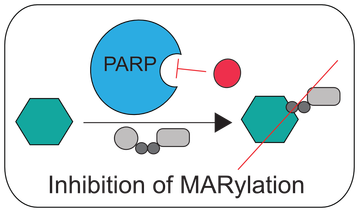
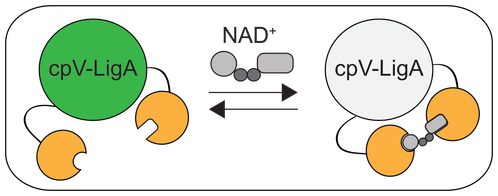
The overarching goal of the lab is to understand the function of ADP-ribosylation in physiology and disease. ADP-ribosylation is a reversible posttranslational modification that is essential for cellular function, yet little information exists regarding relevant protein substrates and target specificity. ADP-ribosylation is catalyzed by a family of 17 enzymes in humans known as poly-ADP-ribose-polymerases (PARP1-16 in humans; also known as ARTDs), which transfer the ADP-ribose moiety from nicotinamide adenine dinucleotide (NAD+) to amino acids on target proteins. The PARP family is sub-classified based on the ability of the individual PARP enzymes to catalyze the transfer of a single ADP-ribose unit (mono-ADP-ribosylation or MARylation; PARP3, 4, 6-8, 10-12, 14-16) or multiple ADP-ribose units (poly-ADP-ribosylation or PARylation; PARP1, 2, 4, 5a, 5b) onto target proteins. Progress in understanding the specific role of a given PARP in cells has been severely limited by (i) the inability to identify the direct targets for individual PARPs in a cellular context and (ii) the lack of selective inhibitors for individual PARP family members. Our lab has developed chemistry-based strategies to address these challenges. Additionally, we have used more conventional methods to uncover new roles for PARPs in neurodevelopment. Lastly, we are generally interested in NAD+ homeostasis, and how fluctuations in NAD+ levels regulate redox as well as NAD+-dependent signaling. To investigate NAD+ dynamics in cells, we developed the first genetically encoded biosensor for quantifying NAD+ levels in cells. Click on the links below to learn more about our work!